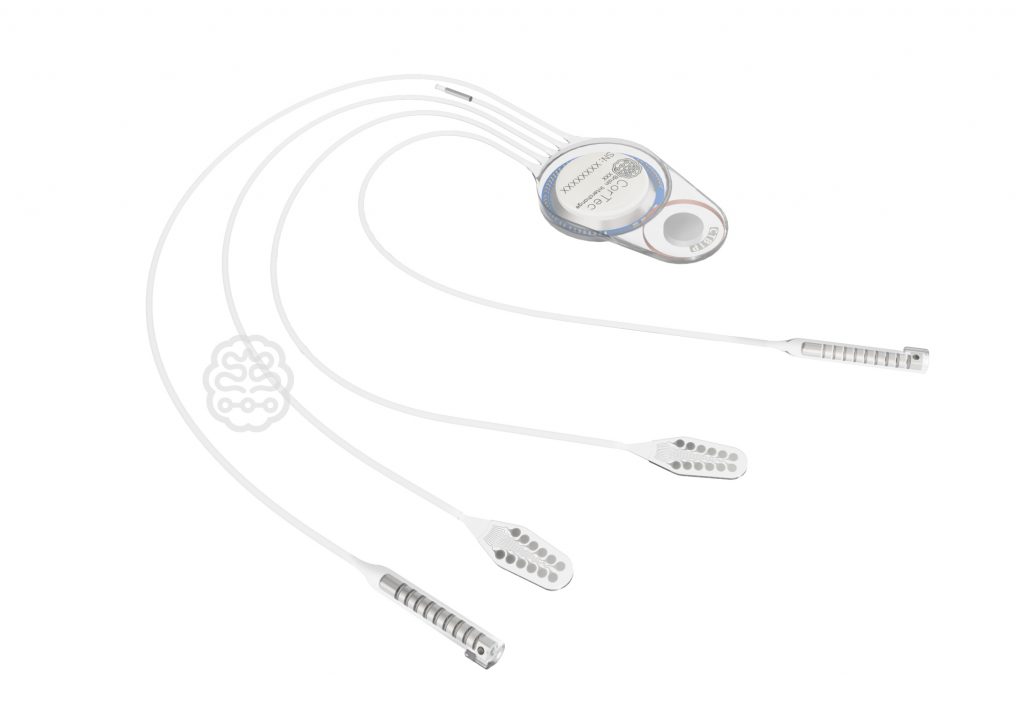
We continue to make progress in the development of our Brain Interchange System. We recently completed our pre-clinical testing and key development milestones. In the next phase of development, we will further refine our technology, and enter the final stages of preparation for our First-in-Human clinical studies.
As the Brain Interchange System enters the next phase, we look forward to collaborating with our U.S. clinical partners to study this novel technology in the treatment of neurogenerative and disabling neurological conditions, disorders, and diseases with unmet or suboptimal clinical solutions.
Claus Freyinger, Chief Regulatory & Clinical Officer of CorTec, says: “We continue to make progress with our pre-clinical work. We remain convinced that the Brain Interchange System is an extremely promising technology to address unmet clinical needs in areas such as stroke rehabilitation, epilepsy, and chronic pain. ”
With our plans and milestones in mind, we will of course provide you with further information on our progress. At INS in Barcelona next month, we will be presenting more details about Brain Interchange and the evaluation kit in several scientific poster sessions.
We look forward to sharing more great news with you – make sure to follow us on LinkedIn to join us on our journey!