German medical technology innovator poised to make waves after implantation of a brain chip by Neuralink in a human trial
As it prepares for the next stage of its groundbreaking device for the treatment of stroke, CorTec GmbH, a pioneering German medical technology company, is poised to make significant strides in the field of neurotechnology.
CorTec is eager to highlight its own advancements in the field, following Neuralink’s recent announcement of the first human implantation of its implantable chip. The company is proud to announce that its cutting-edge Brain Interchange System will enter the first phase of human testing in the coming months. With this significant milestone, CorTec aims to demonstrate its commitment to innovation and improvement of existing therapies.
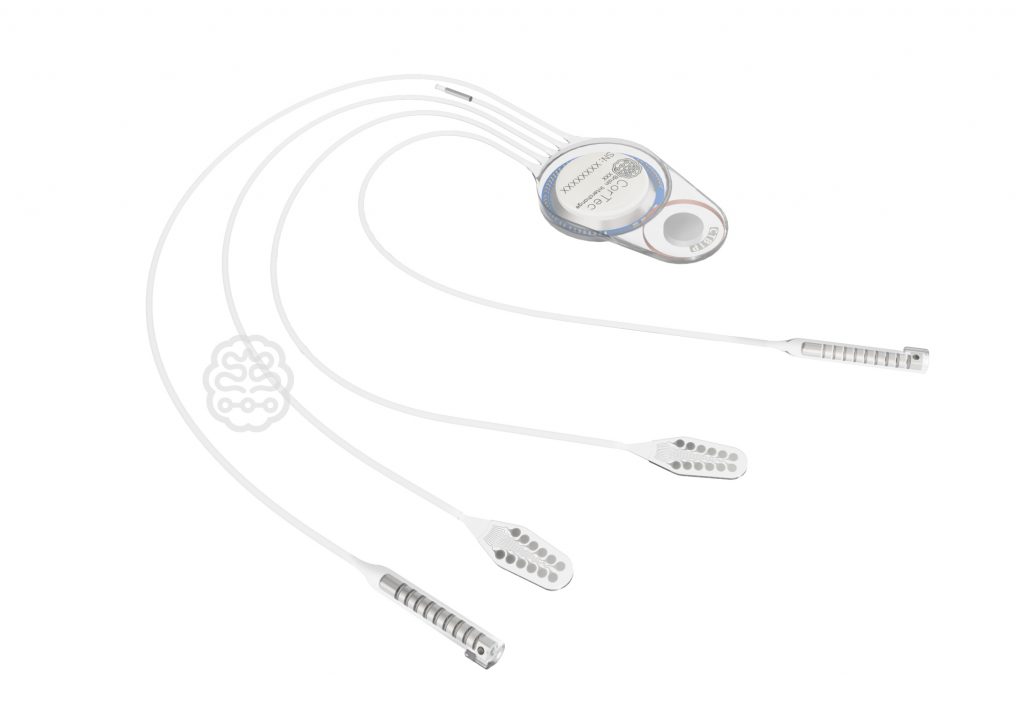
The Brain Interchange System represents a major leap forward in implanted neurotechnology. By continuously monitoring the brain´s state, it accurately assesses each patient’s individual therapeutic needs before precisely delivering optimal electrical stimulation therapy. The system is designed for the treatment of neural network disorders, such as epilepsy, schizophrenia, and Parkinson’s disease. The company invites journalists and members of the press to follow the progress of the Brain Interchange and witness the transformative impact it promises to have on the field of neuromodulation.
In addition, CorTec GmbH is offering exclusive interview opportunities with its Chief Technology Officer, Dr. Martin Schüttler, to provide in-depth insight into the technology behind the Brain Interchange System and the company’s vision for the future of neuromodulation.
Go to the PDF download of this press release (English Version).
Go to the PDF download of this press release (German Version).
Media Contact:
Carolina Remke – Head of Marketing
pr@cortec-neuro.com
www.cortec-neuro.com