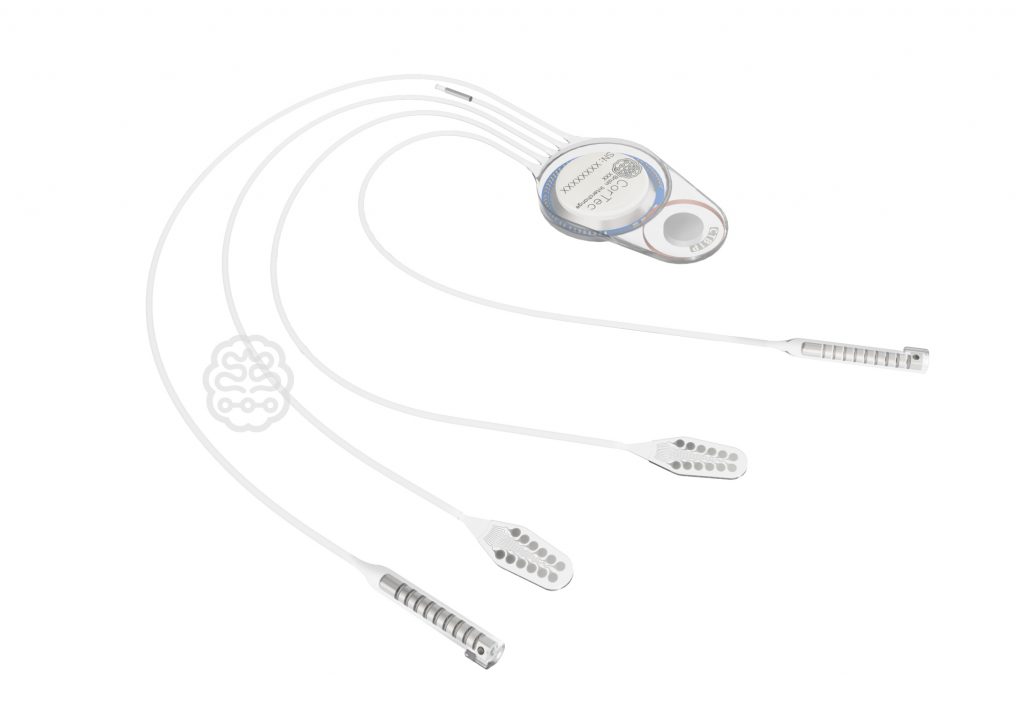
CorTec has announced today that the US Food and Drug Administration (FDA) has approved an Investigational Device Exemption (IDE) application by the University of Washington School of Medicine (UW) involving the closed-loop Brain Interchange Implant System. This clinical study will investigate a novel stroke rehabilitation treatment using cortical stimulation to enhance plasticity within the brain. With the clearance of the Brain Interchange System for human use CorTec is prepared to serve clinicians and research groups with its advanced implant technology to investigate novel treatment options for neurological diseases.
With the Brain Interchange System, CorTec aims to provide a fully implantable closed-loop Brain-Computer Interface (BCI) to clinicians for the investigation of therapies. According to CorTec CTO Dr. Martin Schuettler, this closed-loop functionality provides new possibilities for highly individualized treatments. He states, “The system is capable of interchanging information between biology and technology, between brain and computer. That’s why we call it CorTec Brain Interchange. With our system, we are providing the technological tools that are needed to develop new therapies and brain-computer interface applications.”
With FDA clearance secured, CorTec joins forces with partners in the USA to continue the development of novel therapies. The first IDE study1 involving the Brain Interchange System will be conducted in collaboration with one of the world’s leaders in the field, principle investigator professor Jeffrey G. Ojemann from the University of Washington School of Medicine in Seattle as well as Prof. Steven C. Cramer from the University of California Los Angeles and their respective teams. With funding by the US-American National Institutes of Health (NIH)2, the consortium aims at obtaining initial first-in-human safety data and at the development and evaluation of novel therapeutical rehabilitation approaches for upper limb impairment in stroke patients via direct cortical electrical stimulation delivered by the Brain Interchange System. Enrollment of patients and the first implantation of the neural interfacing system are schedule for the third quarter of 2024.
Assistant Professor Dr. Jeffrey Herron from University of Washington is a co-investigator of the NIH funded study and the engineering lead on the project. He explains the importance of the FDA approval for the upcoming IDE study: “In the United States, all studies involving devices which pose a significant risk require the approval by both the FDA and institutional review board prior to participant recruitment. The FDA review of Investigational Device Exemptions for significant risk device studies is a rigorous process involving the submission of extensive documentation by both UW, the research site, and CorTec, the device manufacturer. The FDA makes their determination for a specific study based upon the details of the study protocol, an extensive hazard analysis, and an in-depth evaluation of the manufacturer’s documents pertaining to the design and testing of the device to ensure that it will perform as needed for the study. The fact that UW and CorTec have now received this IDE approval from FDA is an absolutely critical milestone demonstrating a readiness to proceed towards participant recruitment for this study, pending local UW IRB approval.”
CorTec’s CEO, Dr. Oliver Baertl adds, “We are very excited about the feedback from the FDA! This was an important first step for CorTec to support clinical research in the fast growing neuromodulation and Brain Computer Interface space. We foresee many more studies with our device. The first in human use will be the next milestone for our technology and our company.”
Go to the PDF download of this press release (English Version).
Go to the PDF download of this press release (German Version).
Media Contact:
Carolina Remke – Head of Marketing
pr@cortec-neuro.com
www.cortec-neuro.com
______________________________________________________________________________________
Disclaimer: The research reported in this publication is supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number UH3NS121565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
______________________________________________________________________________________
References:
[1] IDE Application reference G230003/A001
[2] NIH Project 1UH3NS121565-01A1: ” Motor Recovery through Plasticity-Inducing Cortical Stimulation”, in response to RFA-NS-18-023: https://reporter.nih.gov/search/-xvTvG85Ukm-KXyunAWaJw/project-details/10357993