As active implants are dynamically spreading, also the requirements towards their MRI compatibility are increasing constantly. In the framework of the new funding project MR-Implant CorTec is developing new solutions for MRI compatible implants together with the Universtiy of Ulm, the Max Planck Institute for Biological Cybernetics and the company eesy-ic as project partners.
As active implants are dynamically spreading, also the requirements towards their MRI compatibility are increasing constantly. In the framework of the new funding project MR-Implant CorTec is developing new solutions for MRI compatible implants together with the Universtiy of Ulm, the Max Planck Institute for Biological Cybernetics and the company eesy-ic as project partners.
More and more patients receive active implants like cardiac pacemakers, neurostimulators, medication pumps etc. while MRI examinations are becoming the medical standard procedure for an increasing number of indications. At the same time most of the active implants are not or only with strong limitations MRI compatible.
With its Smart Health Initiative the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) faces the challenge to promote innovations in the filed of medical engineering which meet the requirements of patients and medical care systems in the future. The funding program supports the MR-Implant consortium until the year of 2021 to develop new solutions for the MRI compatibility of active implants.
The project which is coordinated by CorTec aims at developing active implant systems which are able to exchange data at high speed, to record as well as to stimulate neural activity while enabling interference-free MRI examinations at the same point in time. The measured data of the implant can then be compared with the results of MRI examination. This way a more precise calibration of the implant system shall be possible as well as an improved therapy which adapts to the momentary needs of the individual patient.
“The possibility to conduct MRI examinations while the implant is operating is extremely advantageous in clinical practice,” said Prof. Dr. Ulf Ziemann, chairman of the Department of Neurology and Stroke at University Hospital Tübingen and member of CorTec’s Scientific Technical Advisory Board. “Being able to precisely locate electrical brain signals enables us to synchronize the placement of the implant with the individual patient’s anatomy. Based on these data we can determine the coordinates of measuring and stimulation accurately so that finally we can implement patient-individual therapy.”
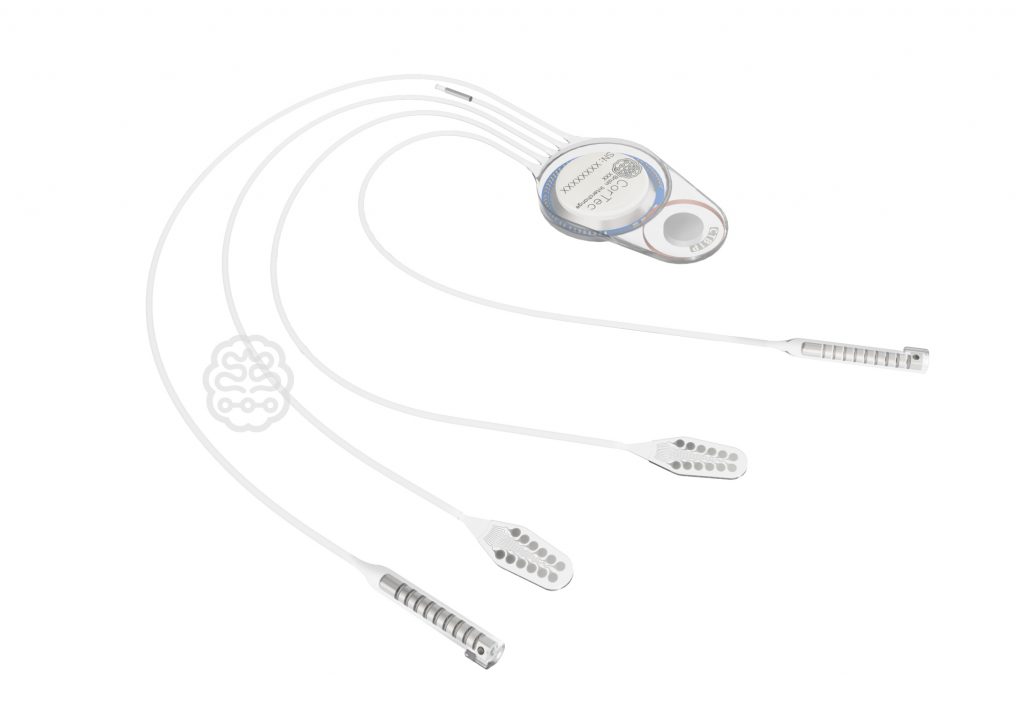
CorTec’s Brain Interchange technology will implement the results of the joint project. The closed-loop implant system can record and stimulate in long-term application. Thus, it enables communication between the nervous system and artificial intelligence – the starting point for the development of innovative personalized therapies.
Learn more about the MR-Implant project
Learn more about CorTec’s Brain Interchange System